The NHS in England currently prescribes weight loss medications in secondary care to patients living with obesity who meet specific criteria. Detailed information about these medications can be found in the National Institute for Health and Care Excellence (NICE) guidelines NG246
Tirzepatide (Mounjaro®)
The National Institute for Health and Care Excellence (NICE) recommends tirzepatide for managing obesity in eligible adults with weight-related health problems like:
- High blood pressure
- Type 2 diabetes
- Sleep apnoea
- Cardiovascular disease
- High blood fat levels
How it works:
Tirzepatide is a weekly injection that helps reduce hunger by making you feel fuller for longer. Your doctor or nurse will show you how to use it.
Important information:
- You must follow a reduced-calorie diet and stay active.
- It’s not suitable if you’re pregnant, planning pregnancy, breastfeeding, or have certain medical conditions.
- It may affect how your body absorbs oral contraceptives or HRT — consider using condoms or HRT patches/gels.
- Let your healthcare team know if you’re having surgery.
Availability:
From 23 March 2025, tirzepatide will be prescribed by specialist weight management services. Initially, only those with severe obesity (BMI ≥ 40, adjusted for ethnicity) and at least four listed health conditions may access it through the NHS. Primary care rollout will be phased and include diet and exercise support.
NICE will review access after 3 years. Check with your local integrated care board for eligibility.
Semaglutide (Wegovy®)
Semaglutide is another once-weekly injection prescribed by specialist services when lifestyle changes haven’t worked. It’s recommended if you have weight-related health issues and a BMI of:
- 35+ (or 32.5+ for some ethnic groups)
- 30–34.9 (or 27.5–32.4 for some ethnic groups) with other criteria
Like tirzepatide, it’s not suitable during pregnancy, breastfeeding, or with certain health issues. Eligibility varies—check with your local integrated care board.
Eligibility & Phased Implementation
As outlined in the NHS Interim Commissioning Guidance, tirzepatide (Mounjaro®) will be introduced through a phased approach focused on clinical prioritisation. Eligibility is determined by BMI thresholds and weight-related comorbidities, including:
- Atherosclerotic Cardiovascular disease – Established atherosclerotic CVD (ischaemic heart disease, cerebrovascular disease, peripheral vascular disease, heart failure)
- Hypertension – Established diagnosis of hypertension and requiring blood pressure lowering therapy
- Dyslipidaemia – Treated with lipid-lowering therapy, or with low-density lipoprotein (LDL) ≥ 4.1 mmol/L, or high-density lipoprotein (HDL) <1.0 mmol/L for men or HDL<1.3 mmol/L for women, or fasting (where possible) triglycerides ≥1.7 mmol/L
- Obstructive Sleep Apnoea (OSA) – Established diagnosis of OSA (sleep clinic confirmation via sleep study) and treatment indicated i.e. meets criteria for continuous positive airway pressure (CPAP) or equivalent
- Type 2 Diabetes Mellitus – May meet criteria for treatment with DM2 alone
Phased Primary Care Cohorts (2025–2028)
- Cohort I (2025-26 ): 4 or more comorbidities and BMI ≥40
- Cohort II (2026-27): 4 or more comorbidities and BMI 35-39.9
- Cohort III (2026-2028): 3 or more comorbidities and BMI ≥40
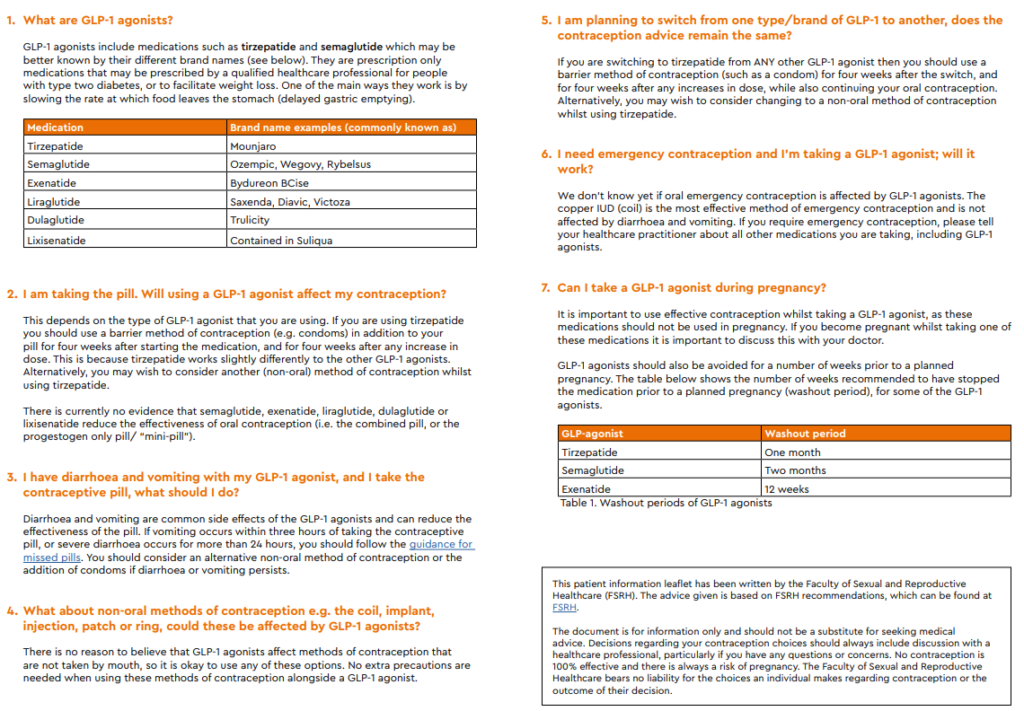
Contraception and weight management injections
For more information, visit the NICE website or speak to your doctor.
